Due to environmental concerns, legislation, and rising energy prices, we're seeing ever-increasing sales of non-fossil powered vehicles, particularly electric vehicles in particular. Too many, however, green cars remain out of reach due to price.
But prices are dropping and will soon reach below that of a comparable internal combustion engine, ICE, car.

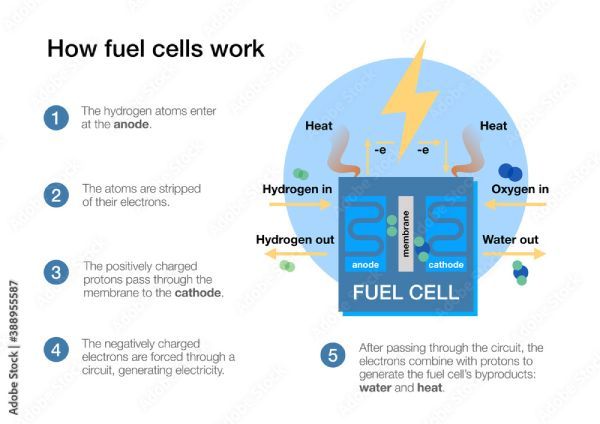
Another method for storing electricity is hydrogen fuel cells. Like internal combustion engine powertrains, a hydrogen engine can quickly be refueled, yet the only emissions are water and the fuel is abundant.
The caveat is, however, that to facilitate the process to produce electricity, a catalyst is required and the catalyst currently used is platinum. A European team led by researchers from the Imperial College of London seems to have found an important part of the answer.
“Currently, around 60% of the cost of a single fuel cell is the platinum for the catalyst. To make fuel cells a real viable alternative to fossil-fuel-powered vehicles, for example, we need to bring that cost down", says lead researcher Professor Anthony Kucernak.
What they've done is that they've substituted platinum with iron. Their method involves dispersing single iron atoms within an electrically conducting carbon matrix, a method that in lab tests has proven to compare favorably to current technology.
"Specifically, we used a unique synthetic method, called transmetallation, to avoid forming iron clusters during synthesis. This process should be beneficial to other scientists looking to prepare a similar type of catalyst”, says first author Dr. Asad Mehmood.
The discovery has potential for other uses as well. For example, it could be possible to use this method to substitute expensive and potentially hazardous chemicals with oxygen. This could benefit, for example, water purification.